Autorom je N. Luptáková.
Abstract
This article discusses the history, chemical properties and a structure of zinc as a pure substance, and also as an alloy. It focuses mainly on a zinc phases and its chemical composition, which are formed in alloys during various technological processes.
Five different kinds of zinc alloys were selected for a description of alloy phases. These alloys have a minimum content of 90% Zn. Accurate assessment of formed phases and their chemical composition is important for suitability assessment in their respective field of industry; for example,removing one or more elements may lead to more valuable use of zinc and its alloys. Zinc alloys are base materials for another products andindustrial processing.
1 INTRODUCTION
In ancient times, before the discovery of a zinc metal, the zinc and copper ore was used as an alloying component in the production of brass; zinc salts have found application in medicine. Brass items were already made in the 3rd century BC in Babylon, Assyria, and in years 1400 to 1000 BC in Palestine. Zinc has been used very early in the production of brass,the metal itself was identified many centuries after. The term zinc was established in the 17th century with the rediscovery of this material. Because zinc is found in nature only in compounds, it was first made from zinc carbonate, zinc salt. Zinc was particularly suited for metal alloying, therefore it was first used as a component in the manufacture of coins.Although the zinc ore was used since the Bronze Age, only much later was found, that it is a separate element; a substance that can not be further divided [5]. Today, zinc is mainly used for steel plating, wall tiling and roofing of buildings, manufacturing of radio covers,sealants, photo-engraved plates and electrodes in zinc-carbon batteries.
2 ZINC PRODUCTION
For a detailed understanding of zinc properties, it’s necessary to know about its crystal structure. Zn crystallizes in the hexagonal structure. This structure has a great influence on the properties of zinc; especially with the monocrystals, the anisotropy of some physical and mechanical properties is very significant. The thermal expansion of zinc in a direction of main axis is 63-9. 10-6, but in a perpendicular direction it’s only 14.1. 10-6. Similar anisotropy occurs with electrical conductivity, but in a lesser extent. For example, molten zinc, which is forming a rough column crystals during crystallization, has a strength in the direction of the main crystallographic axis between 5 to 5.5 hbar, but in a direction perpendicular to the axis of crystallization only about 1.5 hbar. For the same reason,the modulus of elasticity varies depending on an orientation of crystallographic axes in range from 3,500 to 12,400 hbar [3].
Tab. 1 Properties of zinc [1]
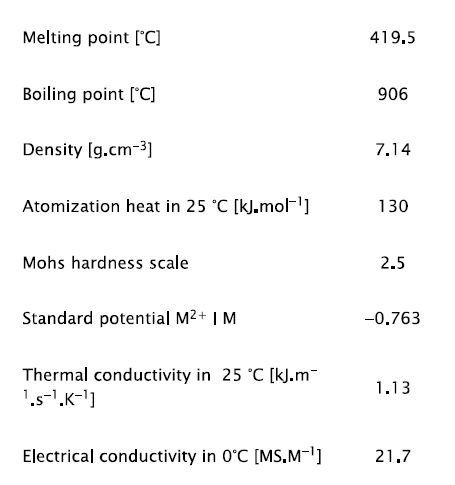
This table shows, that zinc is a transition metal. Ionization energy and the standard potentials show that zinc is chemically less stable [1].
3 EXPERIMENTAL PART
Pure zinc-SHG- SPECIAL HIGH GRADE according to international standards ISO752-2004 and European standards has this composition: min. 99.995 % Zn, max. 0.003% Pb,max.0.003% Cd, max.0.002% Fe, max. 0.001% Sn, max.0.001% Cu,max.0.001%Al and max. total of 6 elements above [4].
It is a relatively soft metal (as for example cadmium), with a good corrosion resistance.Protective oxygen layer, that forms on a surface, has a suitable resistance in atmospheric conditions. Its structure is monotonous and consists of large grains (Fig. 1) -Light optical microscopy (LOM).Grain refinement at the sample surface of SHG zinc is caused by mechanical processing(Fig.2).
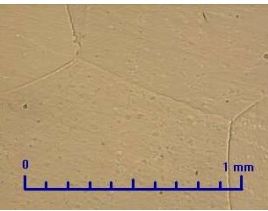
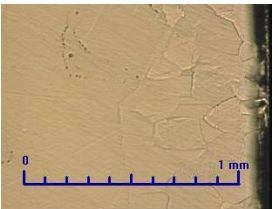
Fig.1 SHG Zinc-50x. Fig.2 SHG Zinc-50x
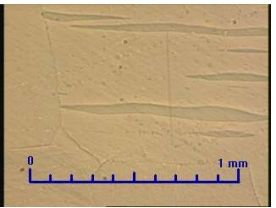
Fig. 3 Unknown phase in the microstructure of SHG Zinc-50x
SHG zinc should contain 99.995 % of Zn; after finding unknown phase from zinc sample (Fig. 3), we did a chemical analysis with BDS method on JOEL JSM-7600F Thermal FE SEM, with these results:
Tab.2 Chemical composition [wt.%] of sample SHG zinc.

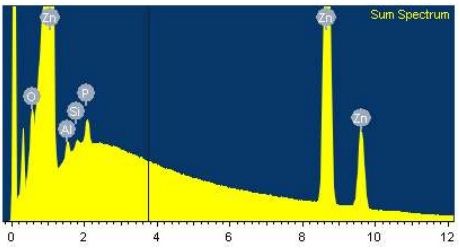
Fig.4 EDAX spectrum of unknown phase in case of SHG Zinc
Analysis (Tab. 2 and Fig. 4) shows, that pure Zn contains Al, Si,S and O in smaller percentages. These phases are formed in metallurgical process during melting or casting.
4 ZINC ALLOYS
Given the fact, that zinc compound is present with other elements in larger percentages,we can call this material an alloy. From all additives in zinc(Fe,Pb,Cd, Sn etc.), the iron has the greatest influence on recrystallization. With the presence of one thousandth of iron,zinc gets recrystallized at room temperature. Zinc with 0.01% Fe recrystallizes at 70 to 100°C.Generally speaking, additives contained in zinc have a significant impact on its hardness.Iron increases the hardness and decreases plasticity of zinc. Zinc with iron forms a solid solution, although its solubility in zinc is negligible (about 0.01% Fe). Moreover, the aloys rich in zinc forms eutectics containing only o.018% Fe, which then forms into the δ phase (solid solution of a chemical compound FeZnj). Because crystals of δ phase (FeZn;) are hard and brittle, this compound cause increased hardness and brittleness of zinc. With the 0.2%of Fe, the zinc is brittle and its further processing is more difficult. Lead, cadmium and tin are easily melting eutectics with zinc; these eutectics are formed at the grain boundaries;the heat processing can cause cracks in zinc. A similar effect has tin and cadmium in zinc. The effect of cadmium in zinc is not as dramatic, because of its solubility (up to 2 %). Harmful effects of lead and tin are even greater, if these additives are simultaneously present; in this case, the ternary eutectics Sn – Zn – Pb is created, which accumulates at the grain boundaries, and melts at 150°C. The lead is added into a specific types of zinc intentionally (polygraphic zinc; up to 1 %), to increase the solubility of zinc in acids for manufacturing of prints [2].To understand the phases, which are forming in the zinc alloys (Zn content min.90%), five types of chemically different zinc were selected (chemical composition is in Table 3); this zinc is also known as a „hard zinc“. Samples were collected and processed from ingots and molds. The structures of these samples correspond to the order of Tab. 3,which are subsequently documented from Fig. 5 to Fig. 19.
Tab.3 Chemical analysis of selected elements in 5 Zn alloys in wt. %
Number of Sample | Pb | Cu | Cd | Ni | Fe | Zn | AI |
1. | 0.0006 | 0.00041 | 0.00033 | 0.0015 | 1.2 | 96.14 | 1.74 |
2. | 1.2 | 0.055 | 0.0005 | 0.008 | 3.3 | 90.5 | 1.2 |
3. | 0.56 | 0.018 | 0.00062 | 0.0035 | 5.5 | 92.63 | 0.52 |
4. | 0.008 | 0.001 | 0.00033 | 0.11 | 0.5 | 99.7 | 0.55 |
| 5. | 0.0037 | 0.0015 | 0.0011 | 0.00031 | 0.0019 | 99.36 | 0.086 |
Fig 5. Sample No.1-100x
Fig 6. Sample No.1-100x

Fig. 7 Sample No. 2-100x
Nonuniform grain (Fig. 5) distribution in the microstructure of sample zinc with a phase formed at the grain boundaries in combination with a number of carbides showing the same tendency. In Fig. 6 we see LOM micrograph of Si containing inclusion. Microstructure in Fig. 7 has mosaic composition of square phases (increased attention was given to this phases in following analysis).
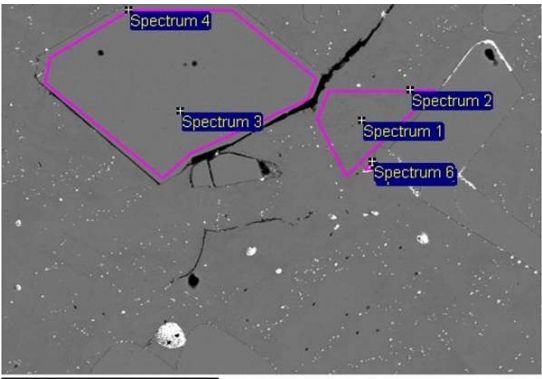
Random structures of zinc alloys with square phases were selected. These phases (Fig.8)and fine particles (contained in phases, or outside of these phases) were analyzed by the method BDS on JOEL JSM-7600F Thermal FE SEM.
100μm
Fig. 8 Sample No. 2-500x
From analysis (Tab. 4) it’s apparent, that selected phases were rich in Fe and Al. We can assume,hat they are Al2O3 and Fe2O3, or one of their complexes.
Tab.4 EDAX spectrum analysis in Fig. 8 (in percentages)
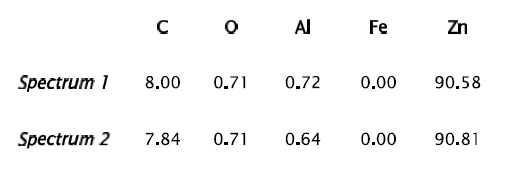
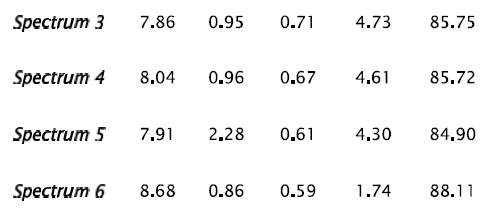
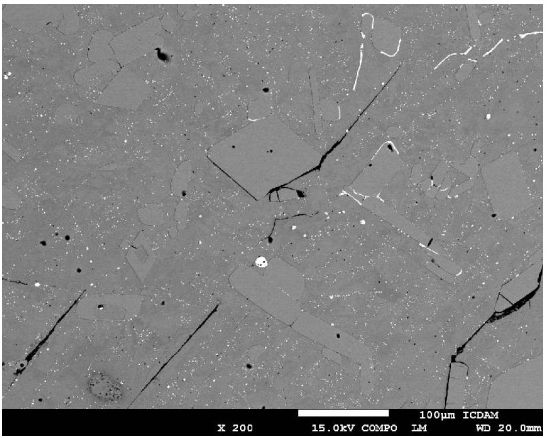
Fig 9 Sample No.2-200x Scanning electron microscope (SEM) micrograph illustrating the crack propagation initiated by the matrix/inclusion decohesion.
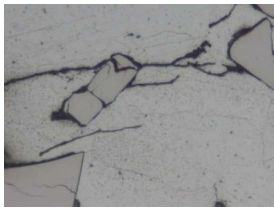

Fig. 10 Sample No. 2-500x Fig. 11 Sample No.2-100x
In Fig 10 – LOM micrograph is showing the crack initiation by the matrix/particle decohesion,particle cracking and the localization of deformation at the tip of the inclusion causing an evident notch effect.
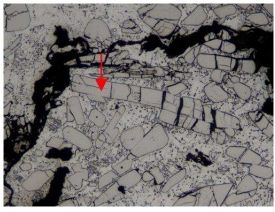
Fig. 11 – Thermal cracks caused by the presence of oxides formed by casting process.
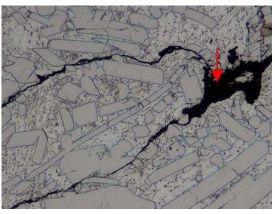
Fig. 12 Sample No. 3-100x Fig. 13 Sample No. 3 -100x
Red arrow means: Fig. 12 – Aggregates with a stone-like morphology.Fig.13- Oxides initiated the thermal cracking. The structure in Fig. 12 and Fig 13 contains about 60 % of the complexes and other phases, and 40 % of Zn. One typical trait of those structures is, that they contain large percentage of oxides (which forms a layer of oxidation film) – black color and mosaic structure can be called a „stone structure.“

Fig. 14 Sample No. 4-500x

Fig.15 Sample No.4-100x
Red arrow means: Crack primarily formed by mold casting of Zn alloy. In sample No.4,the structure was cleaner; it probably contained large tension, which caused de-cohesion of grains (Fig. 14), or local tension was formed (Fig.15).

Fig. 16 Sample No.4-100x

Fig.17 Sample No.4-500x
In sample No. 4, we can see martensite-like structure (Fig 16) and the beginning of a new phase transition (Fig. 17-in red circle).

Fig. 18 Sample No. 5-100x
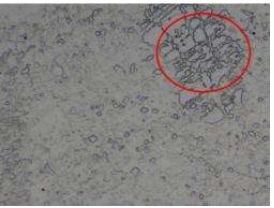
Fig. 19 Sample No.5-100x
Selected structures in red circle represents dendritic structure. Of the structures in No. 5, we can notice the primary crystallization of zinc (Fig. 18), because the structure contains residues of dendritic formations (Fig.19).
CONCLUSION
We can draw several conclusions from the results of microscopic structure and chemical analysis:
·The pure zinc (SHG) and secondary zinc -„hard zinc -(ZnT)“ – is used for the production of zinc products.
·The best option for further processing of zinc metal is to use chemically pure zinc. From financial stand point, it’s better to process ZnT; however this contains more elements.
·For the use of zinc metal for another be the best use of chemically pure zinc. But it is more cost-effective processing of ZnT, which is loaded with great presence of other additives.
·It’s clear that these elements are affectingthe character of structures. Nature of the structure will eventually translate into the quality of the finished product. This means that the presence of impurities and other unsuitable phases will have an impact on the whole technological process.